
|
|
|
Chapter 2: Simple Thermodynamic System: 2-1: Thermodynamic Equilibrium: Thermodynamic coordinates change----->is called the change of state. For isolated system, this system is not influenced by its surroundings, which does not have much application to thermodynamics. So, when thermodynamic equilibrium reached, the system is equilibrium with its surroundings mechanically, chemically and thermally. Thus; thermodynamic equilibrium include the following conditions: States of thermodynamic equilibrium can be described in term of macroscopic coordinates that do not involve the time, i.e. in terms of thermodynamic coordinates. (�y�z���ʤO���Ũt�Ϊ��ѼƧ��ЬO���H�ɶ����ܪ��C) "A system reaches thermodynamic equilibrium", that means, the system is in a state of thermodynamic equilibrium, and this state can be described in terms of macroscopic coordinates that do not involve in time, i.e. in terms of thermodynamic coordinates. (The rest of our discussion concentrate on equilibrium state, not nonequilibrium case.) Every system in thermodynamic equilibrium is a state which can be described by an equation of state (and the only one) which is specifically for "the state", though the equation of state may be so complicated that it cannot be expressed in terms of simple mathematical functions. For instance, ideal gas can be described by PV=nRT, but for real gas in the piston of car engine is not easy to be described by a simple mathematical form, also there exist so many parameters that it is not easy to find the relations among them. Therefore, an equation of state is not a theoretical deduction from thermodynamics but is usually an experimental addition to thermodynamics. Equation of state expresses the results of experiments in which the thermodynamic coordinates of the system were measured as accurately as possible, within a limited range of values. Hydrostatic system: any system of constant mass (i.e. the total number of particles in the system will not exchange with its surroundings) that exerts on the surroundings a uniform hydrostatic pressure, in the absence of surface, gravitational, electric and magnetic effect. Hydrostatic system can be Experiments show that the states of equilibrium of a hydrostatic system can be described with the aid of three coordinates, namely, the pressure P exerted by the system on the surroundings, the volume V, and the absolute temperature T. 2-2 PV Diagram For A Pure Substance: Consider the system of a container about 2 cubic meters in volume from which all the air has been exhausted, and having 1kg of water at temperature 94oC introduced into it, thus the water will evaporate completely and the system will be in the condition know as unsaturated vapor, like point A. At point A, the system is compressed slowly and isothermally, the pressure will rise until there is saturated vapor at point B. If compression is continued, condensation will occur, but the pressure will not change ( this is an isobaric process). The line BC (vaporization line) represents the isothermal isobaric condensation of vapor.
�b�{���I(�G�B��@�s�I)�ɦ��@�ӯS�ʡA�]���O�@�� inflection point�A�ҥH�U������; �Y�b�{���I�]�O�B������ɡ� PV diagram �b�C�Ū�����(�å��b�W�Ϫ��X)���۩T�A��(solid
phase),�P�˦a�P�G�B�𤧶����������Y�]�s�b�T�B��ۤ����C�]�N�O���|���@������BC�������u,�ӳo���u�O�G�B����ɦP�ɤ]�O�T�B����ɡC�o���u�W���I�N�O�T���I(Triple
Point)�C��@���窺�¤��Ө�,�b�T���I�ɪ����O�O611.2Pa,�ūO0.01oC,��n��10-3
m3(saturated liquid)��206 m3(saturated
vapor). 2-3 �ª��誺 Pq ��:In Pq diagram of water, the slope of fusion curve (also called ice line) is negative because the volume become smaller when the ice melt, but for most material the slope is positive because the volume become lager when they melt. The slope of sublimation curve( for water, it can be called frost line) and vaporization curve (for water it can be called steam line) are always positive.
Triple point is the point of intersection of the sublimation, fusion and vaporization curves. �T���I�b Pq �Ϥ��O�@���I�A���O�b PV �Ϥ��O�@���u�C �B�b�ĸѦ����ɡA��n�ܤp(�Ӥj�h�ƪ���h�O����)�A�ҥH�b�W�Ϥ�Fusion curve���ײv�O�t�ȡC�Y
�A���ܦb����n���p�U�A���O�H�ūת��W�[���ܤp�C�Y�O���ȡA�h�ײv�O���ȡA�Y
���ܦb����n���p�U�A���O�H�ūת��W�[���ܤj�ɡA�Y�ĸѦ��G��ɡA��n�ܤj�C 2-4 PVq Surface�� P, V, q �榨�����y�Ъ��۹Ϻ٤��� PVq Surface�C�]���O�@�ӭ��G�٤�Surface�C ��:�b�{�ɷūפ��W�������٤��� Gas phase�A���U�������٤��� Vapor phase�C 2-5 ���A��{��(Equations of State) ���A��{���O���μƾǦ�(�Y�ǥi�Ѳz�ɥX���j�h�Ƨ����g�禡)�y�z�C�@�Ӽ��ʤO���źA��P�BV�Mq�����Y,���O�S���@�ӼƾǦ��O�i�H�ΨӴy�z��Ӭ۹Ϫ��d��C���b�G�A��A(vapor)�M�G��(liquid-vapor
region)���d��A�N���W�L���Q�Ӥ����A�Ҧp�b�C�����]��M����d���i�H�βz�Q�����{���Ӵy�z �䤤 v
���ܨC���ծ��骺��n�C�t�~�b�����T���I����A�i�H�Φ����ӽվ�Ѽƪ�
Beattie-Bridgman equation �Ӵy�z:(�������@�g�礽��) �䤤 One of the most famous of the theoretical equations of states, based on assumptions concerning molecular behavior that are still of use today, is the van der Waals equation of state: This equation holds fairly well in the liquid region, the vapor region, and near and above the critical point. A, B and a, b, c are constant (or fitting parameters) in the above equations. Home Work: 2-6 Differential Changes of StateEvery infinitesimal in thermodynamics must satisfy the requirement that it represents a change in a quantity which is small respect to the quantity itself and large in comparison with the effect produced by the behavior of a few molecules. ���t�ΥѤ@�ӥ��źA�L�p�ܤƨ�t�@�ӥ��źA�ɡA����@�Ӽ��O�Ǯy�Ъ��L�p�ܤơB[�@��H
dx (x �O���O�Ǯy��) �Ӫ���]�άO������O�Ǵ��q�q���L�p�ܤơA�O�������L�p�ܤƪ��j�p�P�����q��ӭȪ��j�p�Ӥ�n�p���h�A���O�n��X�Ӥ��l�惡���q�q���v�T�n�j���h�C�������O�ǬO�Ҽ{���[���t�ΡA�p�Ĥ@���ҭz�C�{�b���ڭ̨ӰQ��²�檺�Ҥl�G�p�G��n�i�H�������O�M�ūת���ơA�]�N�O
�鿱�ȫY��(volume expansivity)�i�H�g��
�������Y�Y��(isothermal compressibility)�i�H���ܬ�
������O�t���O�I�I�O�]���������Y�Y�ƬO���ȡA���Y����n�ܤp�A�ҥH
���W���o�˷L�����覡(
�B�����Y����
�h�ٳo�L�������L���C ���L�����~�@�ӯS�ʡG�YdV�O���L���A�h
�W�����ܥ��L��dV���n���P���|�L���C�]�N�O�]���p���A(�b�U�@�`���A�|���U������) ���[�b�]�B�e�W���@�ǽ��I�G ����W������\�h���褧�]�����O���ܤƨä��ӷP�A�B�H�ūת��ܤƤ]�ܤp�A�ҥH�b�ܤp���ūd���A�]�i�H�����`�ơC���T��G�鵥�����Y�Y�ƣe�A�H�۷ūשM���O�����ܡA�ܤƫܤp�A�G�e�b�����p�U�`�ơA�`�Q�����`�ơC�G�b�n���ɡA�e�A�]�`�Q�����`�ơA�ӳQ����n���~�C ��z�Q����Ө��G 2-7 �ƾ����Y���ѤW�z���ƾ����Y���F�i�H���Ψ��R�y��t�ΡC�Ҧp�ڭ̥i�H�g�X�G �άO �b�W�@�`�ڭ̩w�q�F�鿱�ȫY�ƩM�������Y�Y�ơA �h�]/�e����ȬO�G �����O���ͷL�p�ܤƮɡA��i�H�g���p�U���Φ��G �b��n�O���w�@�w���ɭԡG ���ɷūץѪ�A�ū� �H�U�νҥ��W���Ҥl��W���@�@�өw�q���p��G A Mass of mercury at standard atmospheric Pressure and a temperature of 0oC is kept at constant volume. If the temperature is raised to 10oC, what will be the pressure? �]�� �]=181��10-6 K-1 �e=3.82��10-11 Pa-1 (�b���ūd���A�]�B�e�Q�����`��)
�o�����O�O�۷��j���A�o�]�N�O������ūp�L����|�}���A(�Y�N�q��ūp�m��50oC���������A�h���ūp�N���h�\�ΡC) �W�����Q�O�b����n�����p���U�A���O�����O�����p�S�|�p��O?
2-8 �Q�������u ���䦹�t�Ϊ����O�Ǯy��
|
�䤤 �\ ���u���ȫY�ơA���� 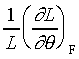 �CA
���u���I���n�AY �����ŷ���Y��(Isothermal Young's Modulus)����
�CA
���u���I���n�AY �����ŷ���Y��(Isothermal Young's Modulus)����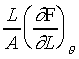 �C�ҥH���A��{����
�C�ҥH���A��{����
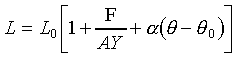 �C
�C
�������i�����T��:
���䦹�t�Ϊ����O�Ǯy��
| 1.�����i�O N/m 2.�������n A m2 3.�z�Q����ū� q |
�b�Q�ת��������ɡA���O�P��n�èS���Q�����t�Ϊ����O�Ǯy�СC��]�O���O�ä����ܡA����n���ܤƥi�H�Q�����C����W�ҩ��A�G��̪��h�����������i�O�ȬO�ūת���ơC�䪬�A��{����
=0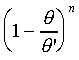 0
�O�b���s�ɪ������i�O�Cq'���b�{���I���ūסA�q�`�Ȧ��X�ת��t�O�C�o��h���������A��{���O
(-w)A
= Const. q w�����R���������i�O�A�Ӫ��Q�o���л\���R���������i�O(�]���o���N�F��������)�C(-w)�٤����������O(Surface
Pressure)�C
0
�O�b���s�ɪ������i�O�Cq'���b�{���I���ūסA�q�`�Ȧ��X�ת��t�O�C�o��h���������A��{���O
(-w)A
= Const. q w�����R���������i�O�A�Ӫ��Q�o���л\���R���������i�O(�]���o���N�F��������)�C(-w)�٤����������O(Surface
Pressure)�C
![]()
�@
�o���b�����W�ά����W���۱m��A���ƻ�H
![]()
�@
Pop Quiz: Removing stains: Some people when they get a greasy stain on their clothes, use a hot iron to remove it. What is the physics underlying this procedure?
Answer: The removal of greasy stains from clothes by ironing them is based on the fact that surface tension decreases as the temperature increases. So if a hot iron is applied to one side of the stained fabric and a piece of ordinary paper is pressed against the other side, the grease is transferred to the paper (or another piece of fabric that absorbs fatty substances)
![]()
�@
�@�Өt�Ϊ��Y�ǩʽ�Y�P��q�L���A�h�o�ǩʽ�ҥN�������z�q�O���j�q�A�p�ūסB���O�B�K���C�Y�Y�ǩʽ�P��q�����A�h���q�O�������q�A�p��n�B��q���C²�檺���դ�k�O�G�N�@�ӥ��Ũt�ε������ⵥ���A��q�]�۵��C��������t�Τ������z�q�A���۵��A���O�b���p�t�άY�Ǫ��z�q�ܦ���t�Ϊ��@�b�A�Ӧ��Ƕq�O�����ܡC�O�����ܪ��q�٤����j�q�A���ܦ���Ӥ@�b���q�٤��������q�C
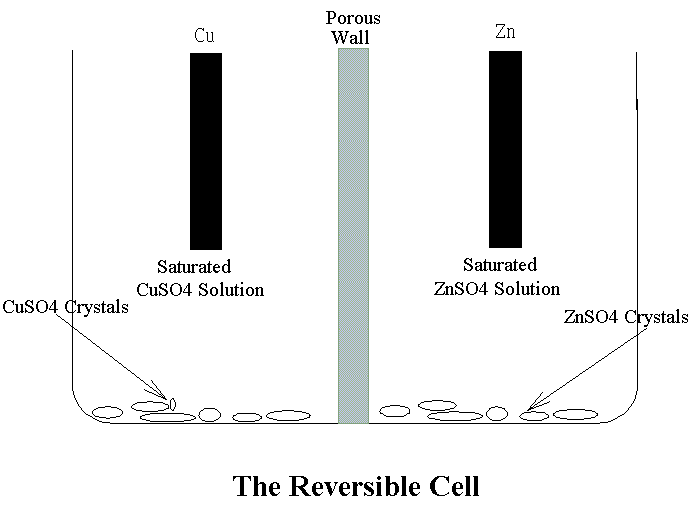
����W�����A�i�f�q���ɷ����N�����a���q�ʡC���ɷ��~���ɽu�Ӧ��������ӾN�����t���ɡA�����p�U(���q�ѻɷ��g�~���ɽu�Ǩ�N��)�G Zn + CuSO4 �С� Cu +ZnSO4 ���ɷ��~���@�q�즨���t���ӾN���������ɡA�����p�U�G Cu + ZnSO4 �С� Zn +CuSO4 �W�z���S�x�ϱo��٤����i�f�q��(Reservible Cell)�C����a�����Gn���ժ��N�����N�|���P��۷����Gn���ժ��ɪR�X�C���ɹq���ܤƥѭ�Ӥ�Zi�ܦ�Zj�h
![]()
�䤤j�����q�l��NF���k�Բı`��(96500Coul)�C���]�i�f�q���O���b�کw���j�����U�B�@�B�S���������X�A�h���O��n�i�H�Q�����A�ӥΥH�y�z���t�Ϊ����O�ǧ��Ь��G
![]()
�@
1. �q�ʶ�e�A��� V
![]()
�@
2. �q��Z�A��� C
![]()
�@
3. �z�Q����ūףc
![]()
�@
���~���u���O�_���ɡA�X���{�H�|�o�ͦӾ�Өt�ΨëD�B�źA�C���~���@�q��p(potentimeter)�ɡA�|���q�y������֨�s�A���ɹq�����q�ʶճQ���ŤF(Mechanical
and Chemical equilibruim)�C�]�u���b�o�ɨt�άO�B����ʤO�ǥ���(because
thermal equilibrium is satisfied too)�C
�Y�q�ѲG�O���M���G�A�B�b�ƾǤ����Ӳ��ͪ��q���ഫ�O�b���ŵ��������ΤU�i��ɡF�h���M���G���@�|�O�����ܡA�]���p���q�ʶդ]�|�O�@�ӱ`�ơC����������M���G�i�f�q���b�������p�U�A�q�ʶեu�O�ūת���ơF�䪬�A��{���O
![]()
t ���ūסA![]() �����20�ɪ��q�ʶաC
�����20�ɪ��q�ʶաC
���q�O�O�@��Ω�q�e���H�W�[�q�e���e�q�q�A�B�O�ѷ��ʤ��l������Ҳզ��C�~�[�q���U�j�A���Ʊj�U�j�C�h
![]()
�䤤 D ��electric
displacement,e0
�O���q�`��(dielectric constant),��P�O�`�q����(total
electric polarization)�A�@���'�W�g��4pP,
P������(Polarization)�CP
�P�q��E���j�p������A�]�P���ʤ��l�����S�ʦ����A�P�ɤ]�O�ūת���ơC�@��Ө����O�M��n�H�ū��ܤƪ��]���i�H�Q�������p�C�ҥH����O�ǧ��Ь�
![]()
�@
1.�q���j��E,���V/m
![]()
�@
2.�q����P,���C��m
![]()
�@
3.�z�Q����ūףc
![]()
�@
���A��{���i�H�g��
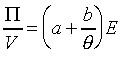
�䤤a,b���`�ơC
���D: �W������ܡA�ūU���q���Ƶ{�U�p�A������?
���Ϫ���b�S���~�[�ϳ������p�U�A�ä��㦳�ϩʡC���O�b�~�[�ϳ��U�A���Ϫ���|�㦳�@�I�I�ϩʡA�B��Ҳ��ͤ��ϳ��P�~�[�ϳ��P��V�C�K�ϩʪ���b�~�[�ϳ����h�ᤴ�㦳�ϩʡA�Ӷ��Ϫ���h���|�A�]���K�ϩʪ��褧�ϤƲv(Permeability)�����Ϫ���j�\�h�C�Y�@���ϲӴθm��ϳ��OH
�����u�ޤ��A���Ϫ���|�]���ϳ�H ���v�T�Ӧ��ϤƧ@�ΡC�]�`�ϯx
M (�q�٬��ϤƲv )�A�h���Ϫ���Ҳ��ͪ��ϷP����
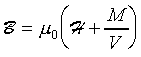
�P�˦a�]���h�b�ҦҼ{�����Ϫ���O�T��A�ҥH���O�M��n�H�ū��ܤƪ��]���i�H�Q�������p�C�G���t�Ϊ����O�ǧ��Ь�
![]()
�@
1.�ϳ��j��H �A��� A/m
![]()
�@
2.�ϤƲv M�A��� A��m2
![]()
�@
3.�z�Q����ūףc
![]()
�@
��![]() �ա�1�ɡA�t�Ϊ����A��{���O�~����{��
�ա�1�ɡA�t�Ϊ����A��{���O�~����{��
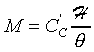
![]() �O�~����{���A��쬰K��m3
�O�~����{���A��쬰K��m3